Blood Advance | Inaticabtagene Autoleucel (CNCT19) in adult relapsed or refractory B-cell acute lymphoblastic leukemia
In Dec 2024, the prestigious * Blood Advances * (IF: 7.4) published a study "Inaticabtagene Autoleucel (Inati-cel,CNCT19) in adult relapsed or refractory B-cell acute lymphoblastic leukemia." co-authored by Professor Wang Jianxiang, Professor Wang Ying, from Chinese Academy of Medical Sciences Haematological Diseases Hospital, and Dr. Lulu Lv from Juventas Cell Therapy Ltd., The 2-year follow-up data demonstrated that Inati-cel perpetuated durable responses with manageable safety profiles in adult relapsed or refractory B-cell acute lymphoblastic leukemia (r/r B-ALL).

Key points:
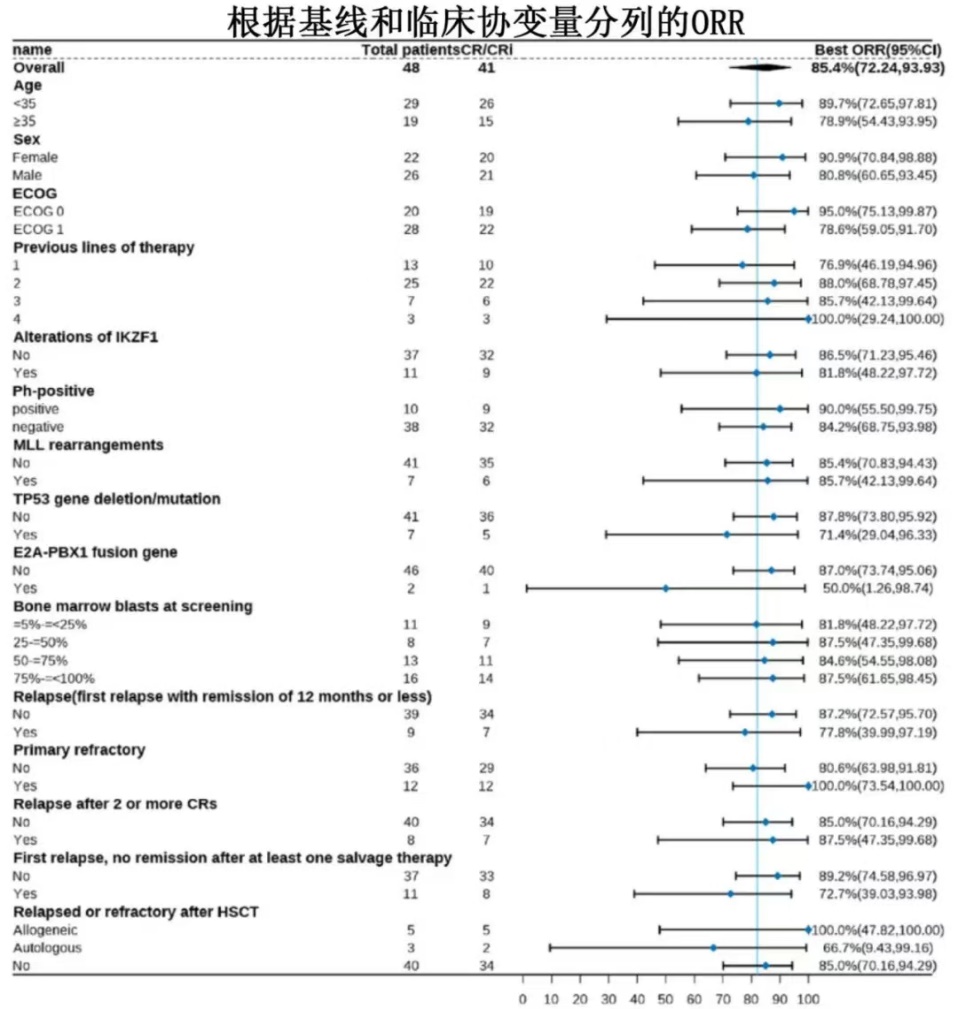
1. Inati-cel induced high and durable responses in r/r B-ALL patients with best ORR achieving 85.4% and median DOR being 20.7 months.
2. At the median follow-up of 23.7 months, Inati-cel showed a manageable long-term safety profile and with no new safety signal finding.
Prior to November 2023, there was no commercial CD19 CAR-T therapies available in China for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (r/r B-ALL), leaving a significant unmet clinical need. In response, Inaticabtagene Autoleucel (Inati-cel), a novel CD19 CAR-T therapy with a distinct scFv (HI19α), was developed and tested in preliminary clinical research showing promising efficacy. Inaticabtagene Autoleucel is the first proprietary CD19-targeted CAR-T product homegrown in China, and the first commercialized CAR-T cell therapy product for B-ALL in China .
We conducted a phase 2, single-arm, multicenter study of Inati-cel in adult CD19+ r/r B-ALL in China. The primary endpoint was the overall remission rate (ORR) at the end of Month 3 by IRC assessment. Forty-eight patients who underwent Inati-cel infusion were evaluated for both efficacy and safety.
Among them, thirty-four patients achieved and maintained remission beyond 3 months, with 3-month ORR of 70.8% (95%CI, 55.9-83.1). The best ORR was 85.4% with all responders reaching minimal residual disease (MRD) negativity.
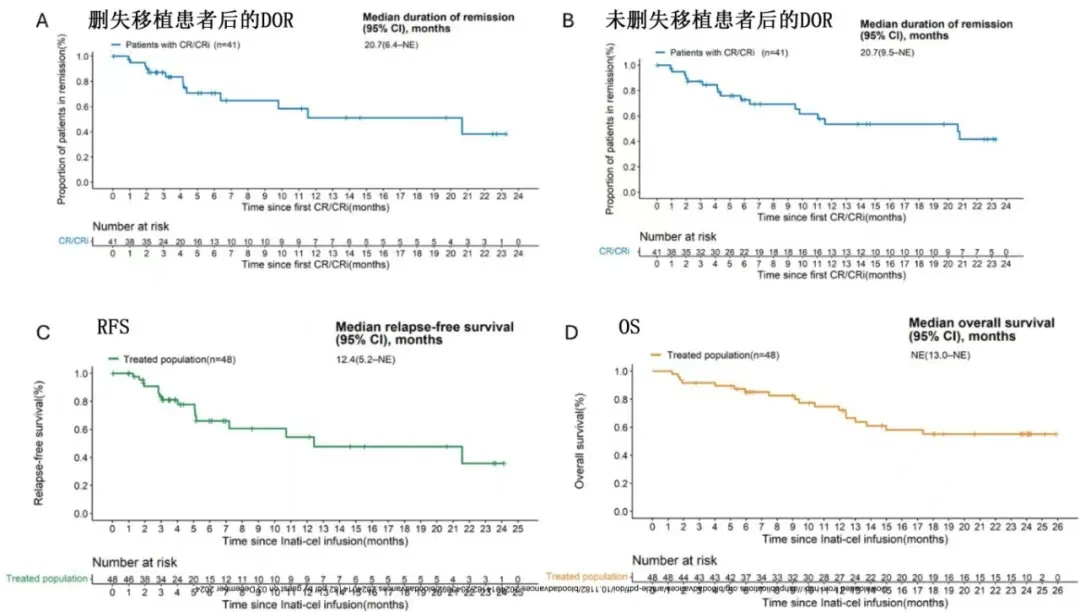
With median follow-up of 23.7 months, the median DOR was 20.7 months (95%CI, 6.4-not reached), and the median OS was not reached (95%CI, 13.0 months-not reached) , with estimated OS rate 55.2% at 24 months.
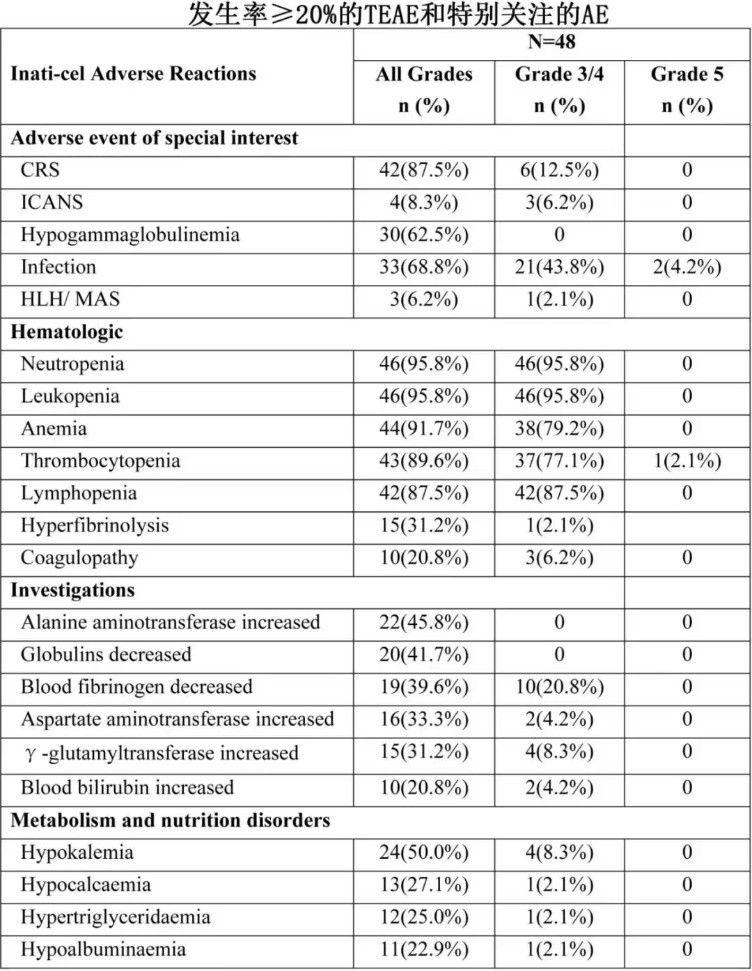
Additionally, grade 3 or higher cytokine release syndrome and neurologic events occurred in 12.5% and 6.2% of patients respectively.